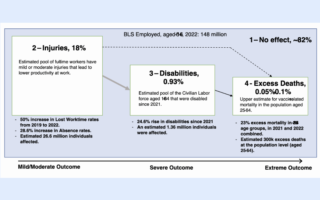
FDA’s CBER Director, Peter Marks, seems focused on seeking power as a global leader promoting gene therapy mRNA shots for all humanity rather than on his US-taxpayer job to protect the safety of vaccines being manufactured for Americans.
He was recently in London to promote gene therapy mRNa shots (deceptively called “Advanced Therapies”) at the World Congress of global elites and has been a major player on the world stage to push “warp speed” use of mRNA gene therapy technology for all vaccines, for humans and animals. Marks, 57, Director of FDA’s CBER Department, has played a key role in nearly every major vaccine-related decision since the United States COVID-19 outbreak began.
“The U.S. response to the pandemic as far as vaccines … was largely his concept,” said Dr. Janet Woodcock, acting FDA director and Marks’ boss. “It was definitely Peter who put the idea together for Operation Warp Speed for vaccines,” she said, which was a way for the government to provide payments and other support for vaccine companies quickly produce enough experimental COVID shots for every American.So, what is his proper job? FDA has its own section in the US Code of Federal Regulation, CFR Title 21, that gives the FDA sweeping powers, especially about inspections, even to the point where they can issue a Consent Decree when a company is delinquent in its manufacturing and quality processes and procedures. That gives the FDA the power to take over running things until remediation has taken place and the failures have been eradicated.
But Marks has not done that to ensure that vaccines for Americans have been manufactured to the highest GMP standards required. To date, Marks has not ordered the FDA inspectors to do on-site inspections of manufacturing plants for America’s COVID “vaccines.” And Marks failed to order the required pre-approval inspections of manufacturing facilities in the US before the experimental COVID shots were launched. Considering the above, we must ask ourselves why Marks is not doing his proper job as Director FDA CBER to protect Americans.
In 2021, Dr. Peter Marks, Director FDA Center for Biologics Evaluation and Review (CBER), spoke at a virtual conference titled: Advanced Therapies, Congress, and Expo 2021. Take a look at the Day 1 Schedule. It is a Potpourri of Big Pharma, academia, research scientists, medics, and business folk. None of them know the first thing about developing complex biologics (that includes vaccines) and the manufacturing supply chain and logistics challenges involved. Nor are any of them holding the manufacturers accountable to the required Good Manufacturing Practices (GMP).
The mantra in biologics manufacture is “the process is the product.” That means every process produces a clinically different product, even if the manufacturing steps appear to be the same. Biologics are living things that react with the vessels, piping, and other equipment in processing. This is well known throughout the industry and is why so few biosimilars are ever brought to market since a competitor must prove their product is clinically similar to the originator product—that is incredibly difficult, often impossible.
Inside Pharma examines these issues and more in today’s Whistleblower Report program.
For more information, check these resources.
Don’t think mRNA is going away any time soon—London will be abuzz with it next month
Investors need to think twice, then twice again, before gambling on mRNA injections
Advanced Therapies, Congress, and Expo 2021
Day 1 Schedule
Moderna’s new booster launch tripped up by production issues at Catalent plant: reports
Rentschler slapped with FDA Form 483, citing lax manufacturing proceduresHedley Rees, based in the United Kingdom. Mr. Rees has been providing consultancy on developing, manufacturing, and distributing drugs that are fully compliant with the GMP requirements of 21 CFR Title 21 since 2005 through his company, PharmaFlow. Before that, he spent 16 years in senior roles in Big Pharma (Bayer) and ten years in biotech (British Biotech, Vernalis, and OSI Pharmaceuticals (now Astellas). Mr. Rees also authored Supply Chain Management in the Drug Industry: Delivering Patient Value for Pharmaceuticals and Biologics for Wiley, NJ, in 2011. His expertise is in the area of anything to do with manufacture through the three phases of clinical trials (and preclinical if required) and ongoing distribution of the commercial supply. Where regulations have been breached, he has a duty to bring this to the attention of regulators, as has been happening with the frozen vials of COVID shots that were shipped only part-way finished.
Hedley joins Truth for Health Foundation Team.
Follow Hedley Rees on his Substack column, INSIDE PHARMA, where he shares all he can to expose the lies and deceptions that are costing lives and jeopardizing public safety. Watch one of his many interviews.
Read more on Facts about SARS-CoV-2 Injections (aka DNA vaccines) and advanced therapy medicinal products (ATMPs)
Watch a Recent interview discussing the violations of manufacturing processes and regulations that are supposed to keep us safe.
For more information, go to Truth for Health Foundation website www.TruthForHealth.org. Download our Vaccine Injury Treatment Guide, file a Citizens Vaccine Injury Report, check our medical and legal resources for help, sign up for newsletter alerts, and join the fight for medical freedom on all fronts.
Your generosity keeps us in the fight for Truth. We still have major battles to defend our freedom on all fronts, especially for our military, who are needing legal defense support. Your contributions will greatly help us support more military families and launch the new year from a position of strength. https://secure.anedot.com/truth-for-health-foundation/donate
Join our Crusade of the Voiceless…WE ARE SILENT NO MORE! Sign up to join our crusade for freedom and receive our alerts!
Image: Reuters